When you pick up a generic pill at the pharmacy, you probably don’t think about how long it took to get there. But behind every affordable medication is a complex, tightly regulated process - and the FDA generic approval timeline is one of the most critical parts. For manufacturers, patients, and healthcare systems, knowing how long this process takes isn’t just helpful - it’s essential.
What’s the Standard FDA Review Time for Generic Drugs?
The FDA’s official target for reviewing a standard generic drug application - called an Abbreviated New Drug Application, or ANDA - is 10 months after the application is accepted. That’s the clock the agency aims to meet for most straightforward generics like tablets or capsules of common drugs such as metformin or lisinopril. But here’s the catch: that 10-month clock doesn’t start the moment a company submits paperwork. First, the FDA does a 60-day filing review to check if the application is complete enough to even begin evaluation. If it’s missing key data - like chemistry details or manufacturing specs - the FDA will send it back without starting the clock. That’s why a well-prepared application matters more than most people realize. In 2025, the FDA’s actual average approval time for standard generics is down to about 35.6 days after the application is accepted - far faster than the 10-month goal. That’s because many applications are approved in the first review cycle, and the FDA has been steadily improving efficiency under the Generic Drug User Fee Amendments (GDUFA). The median approval time? Just 25 days. So why does everyone still say “10 months”? Because that’s the goal - not the average. The 10-month window is a regulatory benchmark designed to give the FDA enough time to handle complex cases. Most simple generics get approved much faster.Why Do Some Generic Drugs Take Much Longer?
Not all generics are created equal. A simple tablet you swallow is easy to copy. But what about a nasal spray, an injectable, or a topical cream with a special delivery system? Those are called complex generics, and they’re where the real delays happen. The FDA requires manufacturers of complex generics to prove not just that the drug works the same way - but that it behaves the same way in the body, down to how it’s absorbed and released. That’s harder to prove than with a regular pill. As a result, these applications often need multiple rounds of review, additional testing, or even physical inspections of overseas manufacturing sites. Data from 2025 shows that complex generics can take anywhere from 6 to 24 months to get approved. One Reddit user reported a nasal spray generic taking 1,087 days from submission to approval. That’s nearly three years. Meanwhile, a standard tablet formulation got approved in just 278 days. The FDA has a dedicated team for complex products, and since 2023, they’ve cut approval times for these drugs by 22%. But the science is still tough. Dr. Janet Woodcock, former head of the FDA’s drug center, once said: “The science of demonstrating equivalence is not straightforward” for these products.What Is a Priority Review - and Does It Apply to Generics?
You’ve probably heard of “priority review” for new drugs. The FDA gives it to medicines that treat serious conditions with no good alternatives. But does it apply to generics? Yes - and it’s becoming more common. The FDA gives priority review to first-ever generic versions of drugs that are in short supply, or where there’s no competition. This speeds things up. While there’s no official timeline published for priority generic reviews, industry insiders say they typically take 6 to 8 months instead of 10. In 2025, the FDA approved 11 first generics in just the first nine months - up from last year. Drugs like epinephrine injection, bosentan suspension, and doxycycline oral suspension were on that list. These aren’t just routine approvals. They’re lifesaving. For example, when a generic version of epinephrine became available, it dropped the price of EpiPens by over 60% in some markets. The FDA also uses its “Commissioner’s National Priority Voucher” program to fast-track certain generics. Under this new initiative, approved applications could be reviewed in as little as 1 to 2 months - a massive jump from the traditional timeline.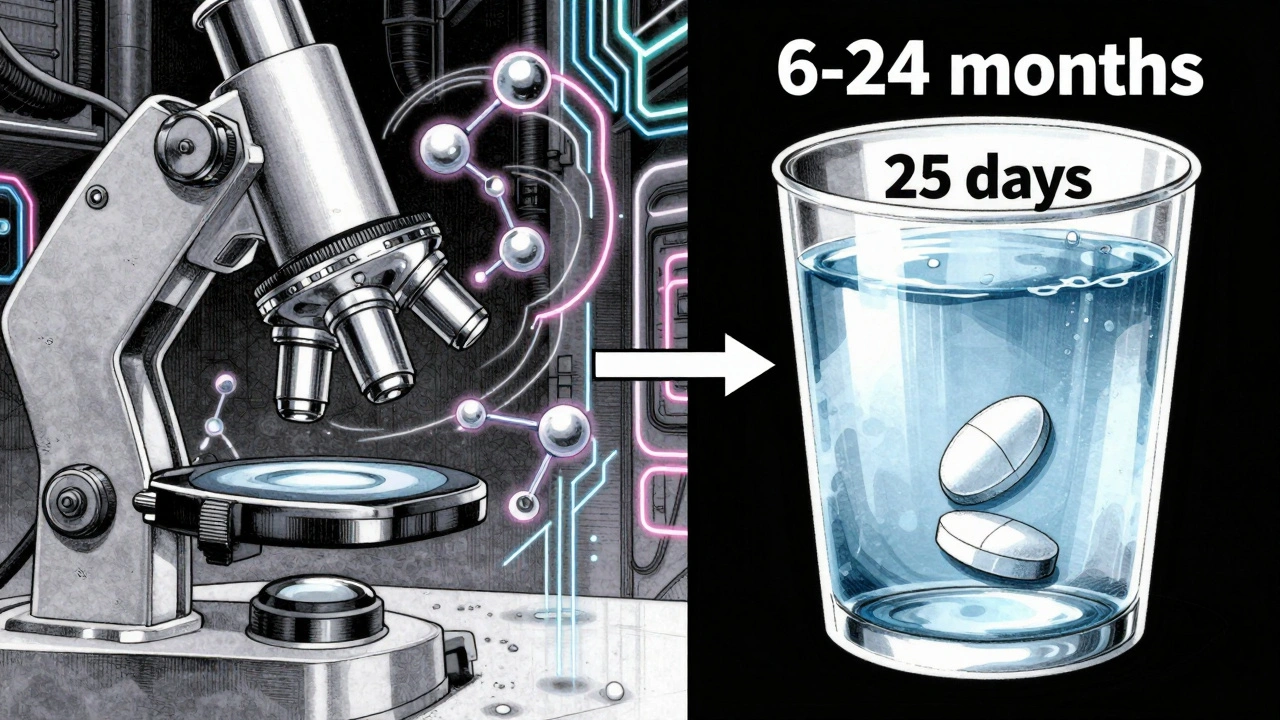
What Happens When the FDA Says “No” - And How Long Does It Add?
The FDA doesn’t approve every application on the first try. In fact, 42.3% of complete response letters - official rejections with feedback - were issued in the first review cycle in Q2 2025. That’s up from 37.8% in 2024, meaning the FDA is getting better at catching issues early. When you get a complete response letter, it’s not a final “no.” It’s a list of what’s missing or needs fixing: maybe the manufacturing process isn’t detailed enough, or the stability data doesn’t match the reference drug. You fix it, resubmit, and the clock resets. Each cycle adds 3 to 6 months to the timeline. If a company gets two or three response letters, that can push approval out by over a year. That’s why top manufacturers like Teva and Viatris invest heavily in pre-submission meetings with the FDA. They ask questions upfront, get feedback, and avoid costly delays. One survey of generic drug makers found that 29% of companies cited “inconsistent application of review standards” as a major frustration. That means two similar applications can get different feedback - which makes planning hard.How Is the FDA Making the Process Faster?
The FDA isn’t just waiting for applications to come in - it’s actively changing how it works. In 2024, pilot programs using artificial intelligence cut review times for standard generics by 15.8%. AI helps sort through data faster, spot inconsistencies in chemistry reports, and flag potential issues before human reviewers even open the file. They’ve also started using “rolling review” for some applications. Instead of waiting to submit the whole ANDA at once, companies can send parts as they’re ready - like submitting the manufacturing section while the bioequivalence study is still running. That saves months. Under GDUFA III (2023-2027), the FDA has set new goals: approve 90% of standard applications on time, and 75% of priority applications on time. By 2027, they aim to hit a median approval time of just 20 days for standard generics and 10 days for priority ones. These aren’t just numbers. They’re changing the market. Generic drugs now make up 90% of all prescriptions in the U.S., but cost only 23% of total drug spending. Over the past decade, FDA efficiency has helped save the healthcare system an estimated $1.7 trillion.
Who Benefits the Most from Faster Approvals?
Patients. That’s the bottom line. When a generic drug hits the market, prices drop - often by 80% or more. A 30-day supply of a brand-name drug might cost $300. The generic? $15. That’s the difference between filling a prescription and skipping it. For people with chronic conditions - diabetes, high blood pressure, asthma - access to affordable generics isn’t a luxury. It’s survival. And when the FDA delays approval, people suffer. In 2025, the FDA prioritized generics for drugs in shortage, including insulin and antibiotics. Those approvals happened faster because the agency knew the stakes. Manufacturers benefit too. Teva, Viatris, and Sandoz - the top three generic makers - now have the shortest approval times because they’ve mastered the process. They submit clean applications. They use pre-submission meetings. They don’t guess what the FDA wants. But the biggest winners? The public. Faster approvals mean more competition. More competition means lower prices. And lower prices mean more people get the medicine they need.What’s Next for FDA Generic Approvals?
The future looks bright - but not without risks. The FDA’s AI tools, rolling reviews, and priority vouchers are working. The agency is on track to meet its 2027 goals. But some experts warn that pushing too hard could hurt quality. Dr. Peter Lurie from the Center for Science in the Public Interest said in August 2025: “Rushing generic approvals without adequate quality assessment could compromise patient safety.” The FDA says it’s using a “risk-based approach” - focusing resources on high-need drugs and complex products. They’re not cutting corners. They’re just getting smarter. Congressional funding is the wild card. GDUFA is funded by fees paid by drug companies - not taxpayer dollars. If those fees drop, or if Congress doesn’t renew the program after 2027, progress could stall. For now, the trend is clear: generic approvals are faster, smarter, and more efficient than ever. And for millions of Americans who rely on affordable meds, that’s not just policy - it’s personal.How long does it take the FDA to approve a generic drug?
The FDA targets 10 months for standard generic drug approvals after accepting the application. But in 2025, the average approval time is just 35.6 days after acceptance, and the median is 25 days. Complex generics can take 6 to 24 months, while priority reviews may take 6-8 months. The FDA aims to cut median times to 20 days for standard generics by 2027.
What is an ANDA, and why does it matter?
ANDA stands for Abbreviated New Drug Application. It’s the form generic drug makers submit to the FDA to prove their product is bioequivalent to the brand-name drug. Unlike new drugs, generics don’t need new clinical trials - just proof they work the same way in the body. The ANDA process is what makes generics faster and cheaper to produce.
Why do some generic drugs get approved faster than others?
Simple generics - like tablets or capsules - are easier to copy and review. Complex products - like nasal sprays, injectables, or topical creams - require more testing and analysis. The FDA also gives priority review to first-ever generics of drugs in shortage or with no competition. These get faster approval.
What happens if the FDA rejects a generic drug application?
The FDA sends a Complete Response Letter listing what’s missing or needs fixing - like incomplete data, manufacturing issues, or bioequivalence concerns. The company fixes the issues and resubmits. Each review cycle adds 3-6 months. About 42% of applications get a response letter on the first try, which is actually a sign the FDA is catching problems early.
Is the FDA using AI to speed up generic approvals?
Yes. In 2024, pilot programs using artificial intelligence reduced review times for standard generics by 15.8%. AI helps analyze chemistry data, spot inconsistencies, and flag potential issues before human reviewers even open the file. The FDA plans to expand this tool to more applications in 2026.
How much does it cost to get a generic drug approved by the FDA?
The user fee for a standard ANDA application in fiscal year 2025 is $138,400. Small businesses and first-time applicants may qualify for fee waivers, but these are rare - only 4.7% of applications received waivers in 2025. These fees fund the FDA’s review process and are required under the Generic Drug User Fee Amendments (GDUFA).
How do faster generic approvals affect drug prices?
Faster approvals mean more competition. When a generic enters the market, the brand-name drug’s price often drops by 80% or more. Over the past decade, FDA-approved generics have saved the U.S. healthcare system an estimated $1.7 trillion. The more generics approved, the lower the overall cost of prescription drugs.


Helen Maples
December 7, 2025 AT 11:10The FDA's 35.6-day average approval time for standard generics is a triumph of regulatory efficiency, but it’s not luck-it’s the result of GDUFA funding, structured timelines, and data-driven review protocols. Companies that skip pre-submission meetings are setting themselves up for failure. The 42.3% complete response rate? That’s not a flaw-it’s a feature. Early detection saves years. If you’re submitting an ANDA and not using AI-assisted pre-check tools, you’re already behind.
Ashley Farmer
December 7, 2025 AT 18:17I’ve seen friends skip insulin because they couldn’t afford the brand. When the generic finally came through after 8 months, it wasn’t just cheaper-it was life-changing. The FDA’s priority reviews for shortage drugs aren’t bureaucracy. They’re mercy in policy form. Thank you for highlighting this.
Sadie Nastor
December 8, 2025 AT 07:10so like… i just found out generic drugs are basically the same but cheaper?? like, mind blown 🤯 i always thought the brand name was better. also why do some take years?? i thought it was just copying a pill??
Nicholas Heer
December 10, 2025 AT 02:0910 months? 35 days? You think this is about science? Nah. This is about Big Pharma’s shadow lobby. The FDA’s ‘efficiency gains’? They’re rushing approvals to satisfy corporate donors. AI? More like algorithmic backdoors for Chinese manufacturing plants. You think they’re checking sterility? Ha. They’re scanning barcodes and hitting ‘approve.’ And don’t get me started on GDUFA-user fees? That’s just legalized bribery. This system is rigged, and you’re all too dumb to see it.
Sangram Lavte
December 11, 2025 AT 06:27In India, we make generics for the whole world. The FDA process is strict, yes, but it’s also fair. If you follow the guidelines, you get approved. No magic. Just discipline. The delay isn’t the FDA-it’s companies submitting half-finished applications. We train our teams for 18 months before even touching an ANDA. Quality isn’t optional.
Stacy here
December 12, 2025 AT 08:33Let’s be real-this isn’t about science. It’s about control. The FDA doesn’t want generics to move fast because then the pharmaceutical oligarchy loses its grip. They let you think it’s about safety, but the truth? They’re gatekeepers. The ‘priority review’? A carrot to keep manufacturers begging. The ‘complex generics’? A convenient excuse to stall competition. And AI? A shiny distraction so you don’t ask who’s really pulling the strings. Wake up. This is corporate theater with a white coat.
Wesley Phillips
December 12, 2025 AT 23:17Let’s be honest-the 10-month target is a relic. Anyone who still cites it is either misinformed or deliberately peddling FUD. The median is 25 days. That’s not a footnote. That’s the new reality. And if you’re still using ‘brand equals better’ as your medical philosophy, you’re not a patient-you’re a marketing demographic.
Ted Rosenwasser
December 14, 2025 AT 12:10AI review tools? Cute. But here’s the real innovation: rolling submissions. I’ve seen companies shave 14 months off timelines by submitting chemistry data while bioequivalence studies are still running. The FDA’s not the bottleneck. The industry’s still stuck in 2010 thinking. If you’re waiting to submit the whole package, you’re already losing.
David Brooks
December 16, 2025 AT 02:25Imagine a diabetic who can’t afford $300 insulin. Then, six months later, a generic hits the market for $15. That’s not policy. That’s redemption. The FDA’s work isn’t glamorous-but it saves lives, every single day. Keep pushing. Keep improving. The people who need this the most? They’re not on Twitter. They’re just trying to breathe.
Oliver Damon
December 17, 2025 AT 15:18The tension here isn’t between speed and safety-it’s between transparency and opacity. The FDA’s increasing use of real-world data, pre-submission feedback loops, and open review criteria is shifting the paradigm from bureaucratic gatekeeping to collaborative science. The 29% of manufacturers complaining about inconsistent standards? They’re not being treated unfairly-they’re being held to the same standard as everyone else. The real issue is the lack of public access to review criteria. That’s the gap we need to close-not the approval time.
Kurt Russell
December 18, 2025 AT 17:30Here’s the bottom line: every day the FDA delays a generic, someone skips their meds. Every week, another family chooses between rent and refills. The 1.7 trillion saved? That’s not a statistic. That’s 1.7 trillion doses of dignity. The FDA’s not perfect-but they’re doing the hard work so the rest of us don’t have to. Keep the pressure on. Keep the funding flowing. And never, ever underestimate the power of a cheap pill.